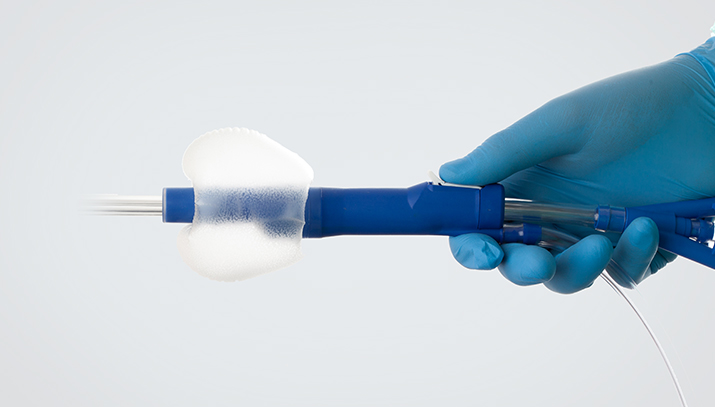
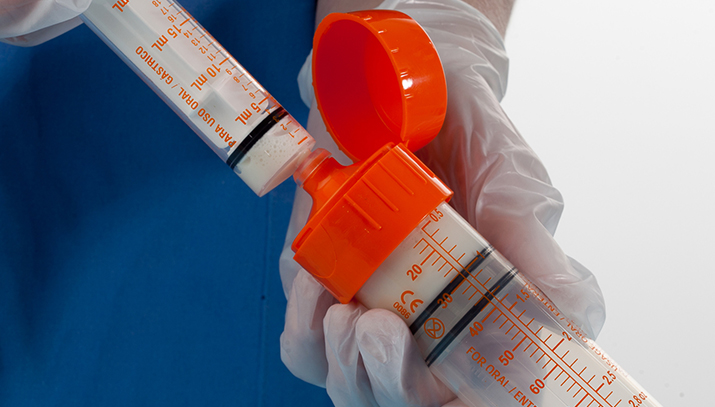
Synecco developed and manufactured two generations of feeding and medication delivery devices for neonates, partnering with a US multinational. Generation 2 enabled our customer’s navigation of the new ISO80369-3 standard, secured valuable IP, and ensured they were first to market with compliant solutions, which accelerated their growth and led to their acquisition.
Delivering human breast milk, feed, supplements and medication to premature infants requires a range of devices and accessories to interact seamlessly, precisely and intuitively for these most vulnerable patients. Add in a new international safety standard and things get even more complex. ISO80369 was introduced to reduce the risk of misconnection between all medical devices, with ISO80369-3 2016 (connectors for enteral applications) the first to change. This disruption presented our customer with both significant challenges and opportunities.
Synecco developed a range of fluid management devices that complied with the new safety standard, solving its required usability and efficacy issues. The range included syringes, tube sets, nasogastric feeding tubes and ancillaries across a range of materials including Purethane, PVC and Silicone. Our design challenges included ensuring these larger more complex connectors were easier to clean, accommodated oral dosing, complied with challenging tolerances and were efficient to manufacture. We succeeded in bringing the new range to market ahead of larger competitors, gaining our customer a leading position in this increasingly competitive field.